
Why ACEi should remain the prefered RAAS inhibitor in HFrEF patients?
In all patients with chronic heart failure and reduced ejection fraction (HFrEF), from NYHA stage II to IV**, evidence-based medical therapies such as betablockers and ACEi*, increase survival, reduce heart failure hospitalizations, improve quality of life and symptoms. According to Guidelines, prescribers must uptitrated them to their maximum tolerated dose to increase patient’s clinical benefits.1
What about the use of the new heart failure treatment, sacubitril/valsartan (ARNi***) which was compared to low dose ACEi (enalapril 10mg bid) in the same patients type ?
The PARADIGM-HF trial effectively compared an ARNi, sacubitril-valsartan full dose, with enalapril low dose in symptomatic patients with HFrEF (EF<40%). Results showed that, overall, ARNi significantly reduced the relative risk of Cardiovascular Death or Heart Failure Hospitalizations by 20% (0.8 (0.73-0.87))2.
Taking a closer look, if this primary endpoint was significant in NYHA class I/II patients (0.75 (0.68-0.84), it was not conclusive in NYHA stage III/IV patients (0.92 (0.79-1.08). The p for interaction was significant confirming the strong statistical difference between the 2 groups (p=0.03). In fact, stage III/IV patients receiving sacubitril/valsartan suffered from an increase in Hospitalisation for Heart Failure (1.09 (0.91-1.31). Interestingly, a second study called LIFE trial3 evaluated sacubitril-valsartan in this type of patients with advanced heart failure NYHA stage III/IV. In the Lancet publication of LIFE trial authors clearly recognized this absence of benefit of PARADIGM-HF trial, “there was no difference between the sacubitril/valsartan and enalapril treatment arms in cardiovascular death or heart failure hospitalisation in the sicker patients with NYHA class III to IV heart failure”. 2,3
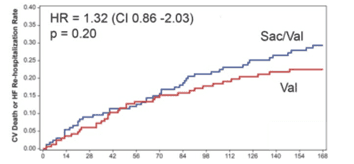
LIFE trial also confirmed those first negative results of PARADIGM-HF. The primary end-point found no significant difference in the evolution of NT-pro BNP levels between sacubitril-valsartan and comparator (0.95(0.84-1.03), p=0.45). Additionally, sacubitril-valsartan did not demonstrate any difference in patients’ number of days alive, out of hospital, and without heart failure events (p = 0.15). There were in fact an increase in events in NYHA III/IV patients receiving sacubitril/valsartan with a 32% increase in Cardiovascular Death or Heart Failure Hospitalization (1.32 (0.86-2.03)). All-Cause Death, Heart Failure Hospitalization, Cardiovascular death, as isolated endpoints, were also not significant. Patients also suffered an increase in hypotension (+17%) and hyperkalemia (+17%, p=0.04).
As LIFE trial was a powerful confirmation of PARADIGM-HF negative results in patients with advanced heart failure NYHA stage III/IV, both highlighting an absence of superiority of sacubitril-valsartan, but higher rate of secondary effects, in comparison with low dose ACEi or full dose ARB, ACEi must remain the preferred RAAS inhibitor for NYHA class III/IV.
Also, as another trial, PARADISE-IM4 confirmed the absence of interest of sacubitril-valsartan in post-MI patients with impaired LVEF<40% on Cardiovascular Death or Hospitalisation for Heart Failure (0.90 (0.78–1.04)4, in comparison with full dose ACEi, what is the real interest of sacubitril-valsartan with only a demonstrated efficacy vs low dose ACEi in stage II NYHA? This also question sacubitril mode of action, in particular on cardiac remodeling.
Thereby, to improve HFrEF patients’ survival and symptoms, the necessary option remains to optimize patients’ treatments by achieving in few steps, an uptitration of ACEi to its maximum tolerated dose, and of course an uptitration of betablocker.
Interestingly, heart rate (HR) control below 70bpm, recognised as providing additional reduction in Cardiovascular Death or Heart Failure Hospitalisation in NYHA stage I/II and in NYHA stage III/IV patients5,6, was not obtained in PARADIGM-HF (mean HR=73) or LIFE trials (mean HR=81), and thus remain a strong additionnal therapeutic objective.
Finally, those three objectives, high dose ACEi, high dose betablocker and control of HR<70 bpm, all recommended by the ESC guidelines, remain the most evidence-based way to reduce symptoms, hospitalizations, and mortality in all HF patients, including stage III/IV patients1,4.
PARADIGM-HF trial 2
Cardiovascular Death or Heart Failure Hospitalisation by prespecified subgroups NYHA I/II and III/IV P for interaction p=0.0335

LIFE trial in advanced Heart Failure III/IV patients
Cardiovascular Death or Heart Failure Hospitalisation
*ACEi: Angiotensin-converting enzyme inhibitors
**NYHA: New York Heart Association
***ARNI: Angiotensin receptor-neprilysin inhibitor
**** NT-proBN : N-terminal (NT)-pro hormone BNP
¹ 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. European Heart Journal, Volume 42, Issue 36, 21 September 2021, Pages 3599–3726,
² Angiotensin-neprilysin inhibition versus enalapril in heart failure. N Engl J Med. 2014;371(11):993-1004. McMurray JJ, Packer M, Desai AS, et al. PARADIGM-HF Investigators and Committees.
³ LIFE TRIAL. Effect of Treatment With Sacubitril/Valsartan in Patients With Advanced Heart Failure and Reduced Ejection Fraction A Randomized Clinical Trial Douglas L. Mann; Michael M. Givertz,; Justin M. Vader & al, AMA Cardiol. 2022;7¹:17-25
⁴ Maurizio Volterrani et al. Effect of Carvedilol, Ivabradine or their combination on exercise capacity in patients with Heart Failure (the CARVIVA HF trial) Int J Cardiol. 2011 Sep 1;151²:218-24. doi: 10.1016/j.ijcard.2011.06.098
⁵ Ivabradine and outcomes in chronic heart failure (SHIFT): a randomised placebo-controlled study
⁶ Heart rate reduction with ivabradine and health related quality of life in patients with chronic heart failure: results from the SHIFT study nger Ekman & al, European Heart Journal (2011) 32, 2395–2404.
To know more : Click here
– For the exclusive use of healthcare professionals SCAC 07/22 DM 206 SERVIER

Keep up to date with our content
Subscribe to our newsletter so that you are always up to date with the news.
